What is the STAR*D Trial?
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial is one of the most influential studies in the field of depression treatment. This large-scale, publicly funded project provides crucial insights into the effectiveness of antidepressants and paves the way for alternative treatments like Transcranial Magnetic Stimulation (TMS).
The STAR*D trial, conducted over several years in the early 2000’s, aimed to evaluate the effectiveness of different antidepressant therapies in people with major depressive disorder (MDD) who had not responded to initial treatment. This study was groundbreaking in its scope, involving 4,041 participants who screened positive for major depression while seeking routine medical or psychiatric care.
The STAR*D trials offered patients up to four treatment stages, aiming to provide guidance in selecting the most effective subsequent treatment for individuals who did not achieve adequate relief from their initial antidepressants (ADs). Each stage encompassed a 12-week open-label trial, with an extra 2 weeks allocated for patients nearing remission. The administration of ADs involved a measurement-based care approach, wherein symptoms and side effects were assessed during each visit. This method guided the adjustment of medication dosages to maximize the likelihood of achieving remission, ensuring that those who did not attain remission were genuinely resistant to the medication.
STAR*D Trial Data of Antidepressant Medication Effectiveness
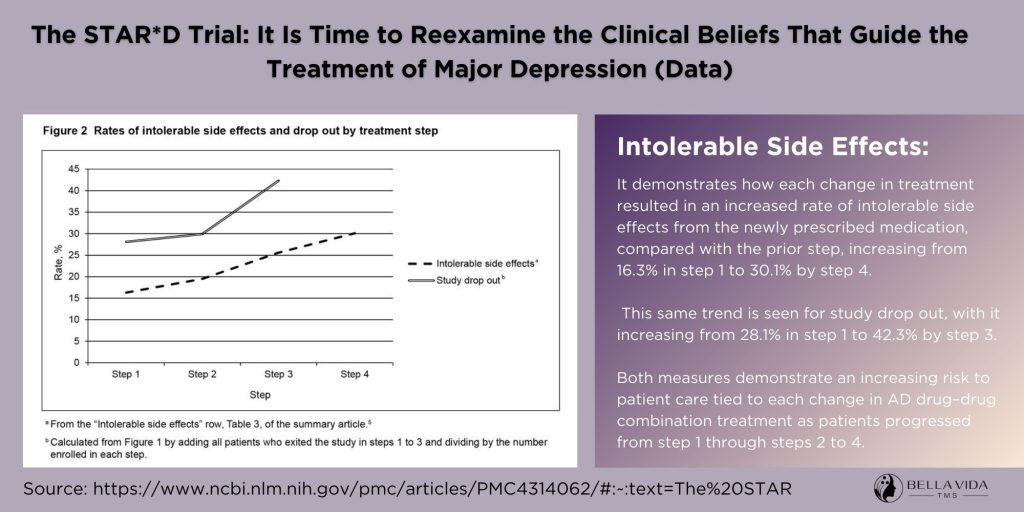
One of the pivotal findings of the STAR*D trial was the diminishing effectiveness of antidepressants with each subsequent treatment step. Initially, patients were treated with a common SSRI (Selective Serotonin Reuptake Inhibitor). Those who did not respond or could not tolerate the medication were moved to the next treatment level, which included switching to a different antidepressant or adding a new medication.
The researchers of STAR*D utilized the unmasked QIDS-SR instead of the protocol-specified HRSD to document outcomes of acute and follow-up care. This assessment was conducted during each clinic visit and monthly via telephone during the follow-up period. The QIDS-SR yielded a more comprehensive dataset, with 690 patients exiting the study in step 1 alone without undergoing the exit HRSD; among them, 152 achieved remission as defined by QIDS-SR. The cumulative percentage of patients achieving remission based on their final QIDS-SR after up to four treatment trials was 45.9%. By step 4, the cumulative percentage of such patients entering follow-up dropped to 37.6%. Despite using 4041 initial participants as the denominator for both calculations, the findings only marginally improved when utilizing 3671 patients as the denominator (50.5% and 41.4%, respectively).

The data provided by STAR*D investigators regarding the sustainability of treatment benefits are even less promising. For step 1, only 17.8% of patients treated with citalopram achieved remission as determined by their last clinic-administered QIDS-SR and did not experience confirmed relapse during follow-up, based on at least one of the 12 monthly telephonic QIDS-SR assessments.
After up to four rounds of antidepressant drug combination treatments, the cumulative rate of patients without confirmed relapse increased only to 23.5%. When dropouts are considered, the durability of treatment effects becomes even less significant.
Merely 2.7% of patients achieved remission as determined by QIDS-SR after up to four rounds of antidepressant drug care and neither relapsed nor dropped out, as indicated by participating in at least one of the monthly telephonic assessments from months 10 to 12 and not exhibiting signs of relapse in any of the 12 monthly administered assessments.
Treating Major Depressive Disorder with Medication
The STAR*D trial highlights the need for alternative depression treatments, especially for those who do not respond to traditional antidepressant medications.
The current landscape of MDD drug treatment primarily revolves around several classes of antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and atypical antidepressants.
Antidepressants are associated with a range of potential side effects that vary depending on the medication and individual factors. Common side effects include nausea, diarrhea, insomnia, drowsiness, sexual dysfunction, weight gain, and dry mouth. Some individuals may experience more severe or persistent side effects that require close monitoring and possible adjustment of the medication regimen.
Antidepressants may carry the risk of more serious adverse effects, such as increased suicidal thoughts or behaviors, particularly in young adults and adolescents. Therefore, close monitoring by healthcare providers is essential, especially during the initial weeks of treatment and whenever dosage adjustments are made.
This is where Transcranial Magnetic Stimulation (TMS) becomes a stronger if not better treatment option for MDD.
TMS: A Promising Alternative to Antidepressant Medication
TMS is a non-invasive, FDA-approved treatment for depression. It uses magnetic fields to stimulate nerve cells in the brain regions involved in mood control and depression. Unlike antidepressants, TMS does not involve medication and is generally well-tolerated, with few side effects.
STAR*D trails struggle to show solid remission rates in the data set suggesting the data is either rounded up or down with implication of false reporting on the criteria of remission.
A complete table of bias and research contradictions is provided in the full article showing data deviations, unapproved off procedure analytical tools, and using data from ineligible patients.
It is important for the scientific community – when reviewing published scientific papers – to report these discrepancies in scientific procedures and data reporting/calculations to the public.
A study published in Harvard Health Publishing showed approximately 50% to 60% of people with depression experienced a clinically meaningful response with TMS during this study.
In 2018 Brain Stimulation academic journal published TMS + psychotherapy resulted in relatively high response (66%) and remission (56%) rates with 60% sustained remission at follow-up.
Connecting STAR*D Findings to TMS:
The results of the STAR*D trial highlight the reality that a significant number of individuals with depression may not find lasting relief from antidepressant medications alone. This realization opens the door for TMS as a viable alternative or adjunct treatment. TMS offers hope for patients who have not benefited from traditional antidepressant therapies, aligning with the need for more personalized and varied treatment approaches as suggested by the STAR*D outcomes.
The STAR*D trial has been instrumental in broadening our understanding of depression treatment. It reveals the limitations of antidepressants and underscores the importance of alternative therapies like TMS. For those struggling with depression, especially those who have not responded to medications, TMS offers a promising, well-tolerated, and effective treatment option.
At Bella Vida TMS, we are committed to providing innovative treatments like TMS, guided by the latest research and tailored to individual needs.
External Reference links
- National Institutes of Health – The STAR*D Trial: It Is Time to Reexamine the Clinical Beliefs That Guide the Treatment of Major Depression
- The STAR*D trial Data : – Indicators of Bias Previously Documented Data Table
- Harvard Health – Transcranial magnetic stimulation (TMS): Hope for stubborn depression
- Brain Stimulation – Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study



